Ozone layer
The ozone layer shields the Earth’s surface from damaging ultraviolet radiation.
What is ozone?
Ozone is a molecule that consists of three oxygen atoms, giving it the chemical formula O3. This contrasts with the oxygen in the air we breathe, which is made up of molecules with two atoms, O2.
Ozone forms naturally when oxygen molecules absorb certain kinds of ultraviolet light and recombine into ozone. It can also be made by electrical discharges. More ozone is made commercially, and it has a wide range of industrial uses.
Ozone is far more reactive than normal oxygen molecules and can have damaging health effects. For this reason, it is classified as an air pollutant when it occurs at low altitude. This happens due to chemical reactions with various ozone precursors found in industrial emissions, vehicle exhausts and other anthropogenic sources. Ozone air pollution tends to be more of a problem in cities than in rural areas.
What is the ozone layer?
The ozone layer is a zone in the stratosphere in which about 90% of atmospheric ozone can be found. It exists between about 15 to 35 km in altitude.
Ozone is produced at this altitude when oxygen molecules absorb ultraviolet (UV) light from the Sun and split into two single atoms. One of these can then react with an O2 molecule to create O3, ozone. This ozone molecule can then absorb further UV light and split back into O2 plus a single atom, allowing the cycle to repeat.
This repeating process absorbs most of the UV light coming from the Sun, meaning only the relatively less-damaging UV-A and some UV-B reach the surface. If it weren’t for the ozone layer the Earth’s surface would not be habitable for life as we know it.
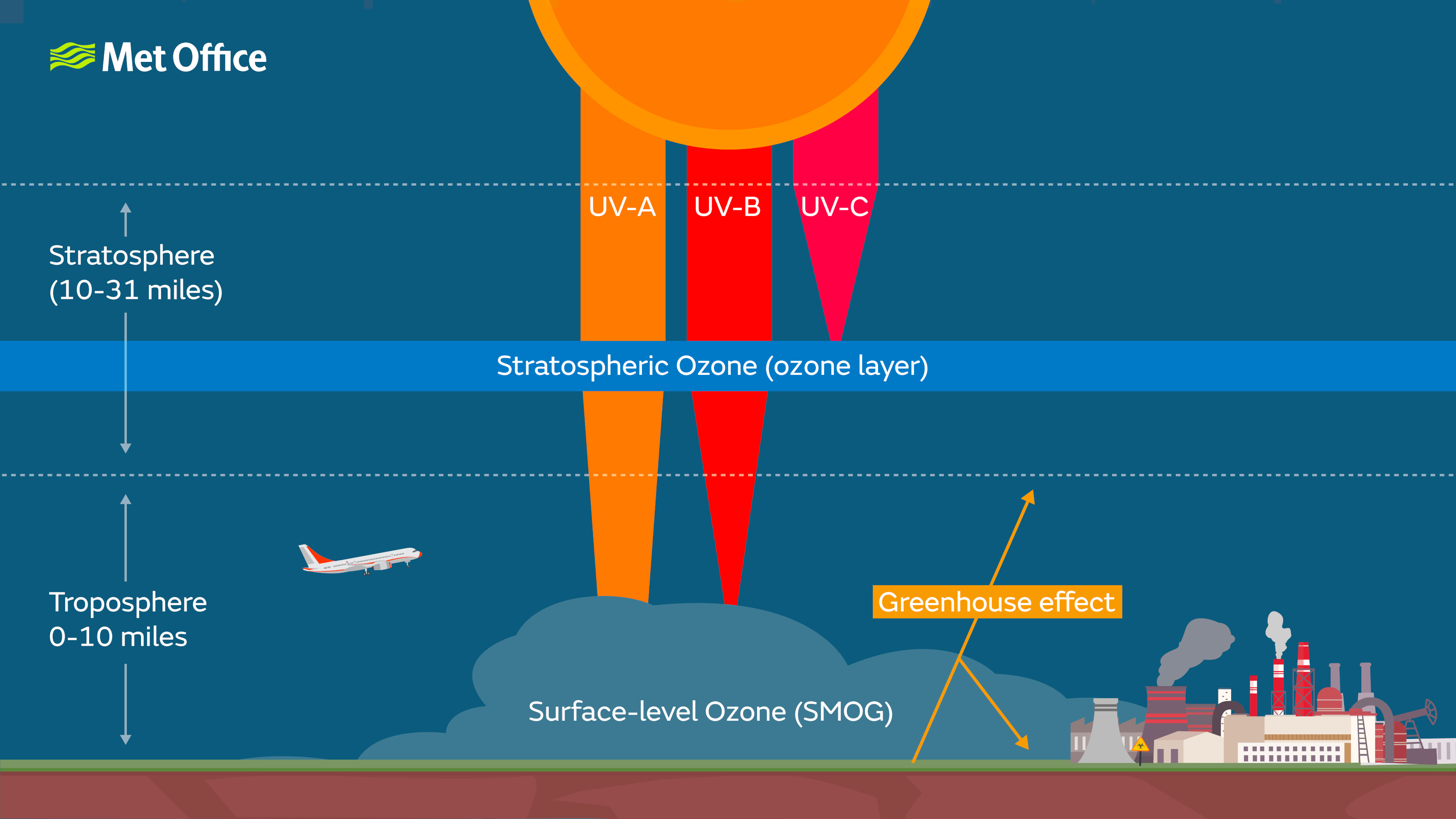
The stratospheric ozone layer filters out the most damaging UV radiation from the Sun.
Although the ozone layer is where most ozone can be found, it is still not very abundant compared to other atmospheric gases. Ozone concentrations in the ozone layer are only about 2-8 parts per million (ppm), far lower than the concentration of carbon dioxide, for example. However, this is still much higher than ozone’s normal concentration at the surface, which is about 0.035 ppm. See our page on Earth’s atmosphere for more information on the gases that make up the air.
The hole in the ozone layer
The so-called hole in the ozone layer, caused by human emissions, has been a major aspect of industrial emissions policy since it was discovered in the 1980s.
Ozone can be destroyed by various chemicals in the atmosphere known as free radicals. These free radicals can be produced in the stratosphere when certain chemicals absorb UV radiation. Such chemicals are known as ozone-depleting substances (ODSs).
ODSs include some naturally-produced substances such as nitric oxide, but the vast majority are made by humans. Most ozone depletion is caused by chlorofluorocarbons (CFCs) and similar compounds. These were originally made for use in air conditioners, refrigerators, aerosol sprays and cleaning products.
CFCs are not very reactive normally, hence why they were so widely used as they were believed to be safe. The problem is this allows them to drift up into the stratosphere unaltered, while natural sources of chlorine and bromine free radicals usually never make it out of the troposphere, the lower layer of the atmosphere, without reacting with something.
Once in the stratosphere, CFCs can absorb UV radiation and create chlorine and bromine free radicals. These free radicals react with ozone molecules, converting them into oxygen molecules. The free radicals are not used up in these reactions, so they can stay in the stratosphere for a long time, destroying many ozone molecules.
These reactions were theorised in the 1970s, before ozone depletion over Antarctica was measured and proven in a landmark study published in 1985. The reason ozone depletion is particularly high over Antarctica is because of polar stratospheric clouds. These form in the lower stratosphere when temperatures get very cold and are common in the Antarctic winter. These clouds encourage the formation of chlorine free radicals, causing most ozone depletion to take place during the winter months.
This cloud effect on stratospheric chlorine was proven by an expedition to collect data in Antarctica in 1986-87. The large amount of ozone depletion was a surprise to the scientific community and galvanised policymakers to address the issue.
Ozone depletion can lead to a wide variety of negative impacts on animals and plants. The reduced absorption of UV radiation in the atmosphere can lead to increased incidence of skin cancer and cataracts, as well as negative impacts on natural communities.
As a result of the scientific findings and the possible severe impacts of ozone depletion, governments around the world began passing laws reducing or banning the production of CFCs and other ODSs. This culminated in the Montreal Protocol, agreed in 1987, which has now been signed by every member state of the United Nations. Signatories to the Montreal Protocol have agreed to phase out ODSs and replace them with less-damaging substances.
Since the passing of the Montreal Protocol, the emissions of ODSs have fallen to a fraction of their levels in the early 1980s and the ozone layer has begun to recover. The hole in the ozone layer over Antarctica still exists, but it has been slowly shrinking over the past two decades thanks to concerted international action.


